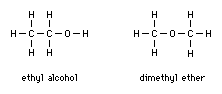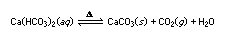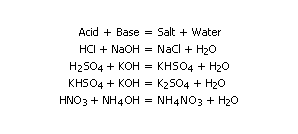Буквы латинского алфавита, обозначающие название элементов, читаются со-
гласно английским названиям букв алфавита.
- Знак + читается plus, and, together, with, react with.
- Знак — обозначает одну связь или единицу родства и не читается.
- Знак = читается give, form или produce.
- Знак → читается give, pass over to lead to.
- Знак ↔ читается forms and is formed from.
-
Цифра перед названием элемента обозначаeт число молекул.
Примеры:
C + O2 → CO2
1 atom of carbon reacts with 1 two-atom molecule of oxygen and produces 1
molecule of carbon dioxide.
2H2 + O2 → 2H2O
a) Two molecules of H two plus O two give two molecules of H two O.
b) Two two-atom molecules of hydrogen react with 1 two-atom molecule of oxygen
and produce two molecules of water.
N2 + 3H2 ↔ 2NH3
a) N two plus three molecules of H two form and are formed from two molecules
of NH three
b) 1 two-atom molecule of nitrogen plus three two-atom molecules of hydrogen
form and are formed from two molecules of ammonia.
Na2CO3 + CaSO4 → Na2SO4 + CaCO3
a) Na two CO three plus CaSO four form Na two SO four plus CaCO three
b) The sodium (Na) and the calcium (Ca) switch
c) The sodium combines with the sulphate radical (SO4), forming sodium
sulphate (Na2SO4) which dissolves in water.
d) The calcium сombines with the carbonate radical (CO3), forming calcium
carbonate (CaCO3).
e) Calcium carbonate does not dissolve in water, and so settles to the bottom
of the solution.
Знаки + или - стоящие в левом верхнем углу, обозначают положительную и
отрицательную валентность иона.
Пример: H+ a) hydrogen ion
b) univalent positive hydrogen ion
Сu ++ divalent positive cuprum ion
Al +++ trivalent positive cuprum ion
Cl - a) negative chlorine ion
c) negative univalent chlorine ion
Now read the rules of nomenclature of the chemical compounds in English.
Rules of nomenclature for inorganic compounds.
ACIDS.
Acids composed of hydrogen and one other element are called binary or hydro-acids. They are named from the second element and always begin with the prefix hydro- and end with the suffix - ic:
H Cl H Br
hydro-chlor-ic hydro-brom-ic
Many acids contain oxygen in addition to hydrogen and another element and are called ternary or ox-acids. Such acids are named after the element other than hydrogen or oxygen and with the suffix - ic:
H2SO4 H3PO4 H2CO3
sulfur-ic phosphor-ic carbon-ic
If the same three elements unite to form more than one ternary acid with the greatest number of oxygen atoms ends in -ic, while the acid with the lowest number of oxygen atoms ends in -ous:
HNO3 HNO H2SO4 H2SO3
nitr-ic nitri-ous sulfur-ic sulfur-ous
BASES.
Bases are named by starting with the name of metal and ending with the word hydroxide. E.g. NaOH is sodium hydroxide, KOH is potassium hydroxide.
SALTS.
Normal salts that are formed from binary acids are named by starting with the name of the metal or ammonium radical and ending the name of the non-metal with the suffix -ide. E.g. sodium chloride for NaCl, ammonium chloride for NH4Cl, potassium bromide for KBr. If the metal has more than one valence, the endings -ous and -ic are used after the name of the metal. A salt in which the metal has lower valence contains the suffix -ous, while the salt of the metal with the higher valence uses the suffix -ic.
The names of salts derived from ternary acids depend on the name of the acid. If the name of the acid ends in -ic, the name of the salt ends in -ate:
- HNO3 - nitric acid / NaNO3 - sodium nitrate
- H2CO3 - carbonic acid / K2CO3 - potassium carbonate
If the name of the acid ends in -ous, the name of the salt ends in -ite:
- HNO2 - nitrous acid / NaNO2 - sodium nitrite
- H2SO3 - sulfurous acid / BaSO3 - barium sulfite
Task: - write any 20 formulas and read them
- write any 5 equations and read them
Elements and Compounds
More than 100 chemical elements—substances that cannot be decomposed or broken into more elementary substances by ordinary chemical means—are known to exist in the universe. Russian chemist Dmitry Ivanovich Mendeleyev and German physicist Julius Lothar Meyer independently developed the periodic law of the chemical elements at about the same time in the late 19th century. Both discovered that arranging the elements in order of increasing atomic mass produced a table of chemical properties and reactivity patterns that were regularly repeated. This phenomenon—known as the periodic law—is most often represented in the periodic table of the elements
Hydrogen, oxygen, chlorine, sodium, and iron are examples of elements. Elements cannot be resolved into simpler substances by ordinary heat, light, electricity, or attack by other substances. It requires either special equipment, such as a particle accelerator, or temperatures like those in the interior of the sun. Ninety elements are known to occur in nature, and 22 more have been made artificially. Out of this limited number of elements, all the millions of known substances are made. Abbreviating the names of the elements is often convenient. For each element, a symbol has been chosen that consists of one or two letters. The symbols are derived from the names of the elements; for example, H stands for hydrogen. The abbreviations are not always derived from the English names, however. The symbol Fe for iron comes from the Latin ferrum, and W for tungsten comes from the German wolfram.
Salt, water, iron rust, and rubber are examples of compounds. A compound is made up of elements, but it looks and behaves quite differently, as a rule, from any of its component elements. Iron rust, for example, does not look and feel like its components: oxygen gas and iron metal.. This individuality of properties, as well as other qualities, distinguishes a compound from a simple mixture of the elements it contains. Another important characteristic of a compound is that a compound has a definite, invariable composition, always containing the same elements in the same proportions by weight; this is the law of definite proportions.
Exercise 1. a) Read and translate the words. Learn them.
Substance, universe, physicist, atomic mass, heat, equipment, occur, iron rust, rubber, contain, composition, weight
b) Write the unknown words down into your dictionaries.
Exercise 2. Ask three questions to each of the passages. Let your group mates answer them.
Exercise 3. Retell the text.
Exercise 4. Give brief characteristics of the elements that you have met in the text. Your description may include: atomic number, atomic mass, group, period, row, chemical and physical properties.
Chemical bonds
Elements that do not have a noble-gas configuration (a stable configuration) try to attain such a configuration by entering into chemical reactions. If atoms are no more than a few electrons away from a stable configuration, they generally attain it by losing or gaining electrons to form electrically charged particles called ions. Positively charged ions are called cations, and negatively charged ions are called anions. The electrons in the outer shell of an element are called valence electrons. Valence electrons are available to form bonds with other atoms. Groups of elements with similar electron configurations behave in a similar way in chemical reactions, so they have similar chemical and physical properties and are called families. The periodic table shows how elements can be grouped into families.
Ionic bonds
Oppositely charged ions have a strong mutual electrostatic attraction when brought together, but, if brought too close, the electron clouds repel each other. Thus, a pair of mutually attracted ions will maintain a certain distance from each other. This distance is called the bond length, and the electrostatic attraction of the ions constitutes an ionic (or electrovalent) bond. Ionic bonds are very common and are exemplified by table salt, in which a sodium ion attracts a chloride ion to form Na+Cl- or, as usually written, NaCl. The total charge on each combination of ions, NaCl and CaCl2, is neutral, or zero.
Covalent bonds
Another common type of bond, the covalent bond, results when two atoms share one or more pairs of electrons in an attempt to fill their outer shells and become more energetically stable. The atoms are held together by the mutual electrostatic attraction between the protons in their nuclei and these electrons. The bonded atoms form a stable unit called a molecule. Covalent bonds tend to form when the bonded atoms have nearly the same attraction for electrons; ionic bonds form when the electron-attracting power of the atoms differs markedly. Some metals form both ionic and covalent bonds. A convenient rule for remembering whether the bond between two elements is likely to be ionic or covalent is that if one element appears on the left side of the periodic table and the other on the right, the bond is ionic.
Vocabulary
Configuration, to lose, to gain, positively charged/ negatively charged, charge, ion, cation, anion, outershell, valence, oppositely charged, electrostatic attraction, covalent bond, electron-attracting power.
Do you have anything to add? What else can you say about types of bonding?
Speak about other types of bonds that you know.
Chemical formulas
To show the atomic constituents of a compound, two or more chemical symbols are put together with subscripts, giving formulas. Since molecules are made up of atoms in simple whole-number ratios, molecular composition can be expressed by means of element symbols, with subscript numbers used to indicate the relative number of atoms of each element. Thus water, with molecules composed of two atoms of hydrogen and one atom of oxygen, is written as H2O; hydrogen peroxide, with two atoms of each of these elements, is H2O2. The formula CO stands for carbon mon oxide (one atom of carbon and one atom of oxygen), and CO2 stands for carbon di oxide (one atom of carbon and two atoms of oxygen). The formula FeSO4 (one atom each of iron and sulfur and four oxygen atoms) stands for ferrous sulfate. The formula Fe2(SO4)3 represents another sulfate of iron, ferric sulfate, which contains three sulfate ions (SO4) and two ferric ions (Fe). When groups of atoms combine as a subunit, the subunit is usually treated as a single symbol. For example, the sulfate group, SO42-, is treated as a single symbol (included in molecules such as Al2(SO4)3, aluminum sulfate), as is the nitrate group, NO3- (included in molecules such as NH4NO3, ammonium nitrate). Ions are represented by adding the electric charge as a superscript. Thus Na+ represents the sodium ion (loss of one electron, or one less electron than Na), Cl- the chloride ion (addition of one electron, or one more electron than Cl), SO42- the sulfate ion (addition of two electrons), Fe2+ the ferrous ion (loss of two electrons), and Fe3+ the ferric ion (loss of three electrons).
Structural formulas
Structural formulas show how atoms are connected together, so that different compounds containing equal numbers of the same elements—known as isomers—can be distinguished from each other. Examples of isomers are ethyl alcohol and dimethyl ether. Both share the simple chemical formula C2H6O. Although ethyl alcohol and dimethyl ether both contain equal numbers of atoms of the same elements, the atoms within the respective molecules are arranged differently, producing two different compounds. These differences can be shown by structural formulas, which show how atoms within a molecule are arranged by using lines to represent chemical bonds:
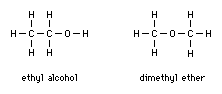
Exercise 1. Repeat the rules of reading chemical formulae and equations. Read the following equations.
a) NaOH + HCl = NaCl + H2O
b) 2Na + 2H2O = 2NaOH + H2
c) Li2O + H2O = 2LiOH
d) CuCl2 + 2KOH = Cu(OH)2 + 2KCl
e) 2HCl + Fe(OH)2 = FeCl2 + 2H2O
f) 2HNO3 + CaCO3 = Ca(NO3)2 + CO2 + H2O
g) 2CH4 = C2H2 + 3H2
Esercise 2. Speak on the following:
1) subscripts and superscripts; give your examples
2) the purpose of structural formulae.
Chemical equations
Chemical reactions can be expressed through equations that resemble mathematical equations. The reactants (the substances that are combined to react with one another) appear on the left side of the equation, and the products (substances produced by the reaction) are written on the right side of the equation. The reactants and products are typically connected by an arrow (→) or various types of double arrows. The single arrow shows that a reaction only proceeds in the direction indicated, while the double arrow indicates that a reaction can proceed in either direction (that products are also reacting with each other to reform reactants). Thus, the equation 2Cl ⇄ Cl2 indicates that two chlorine atoms react to form a molecule of chlorine and that a reaction can also take place in the reverse direction. This reaction, like all reactions, is affected by conditions such as temperature. In the case of 2Cl ⇄ Cl2, the reaction goes to the right at room temperature (25° C; 77° F) and to the left only at much higher temperatures (about 500° C; about 932° F). Sometimes the condition under which a reaction takes place is written over or below the arrow(s).
The Greek letter delta, Δ, is sometimes used to indicate that the reactants must be heated for a reaction to take place. An equation may also show the physical state of the reactants and products by using the letters g, l, s, and aq to stand for gas, liquid, solid, and aqueous solution, respectively. Thus, the equation
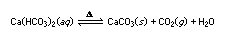
indicates that when a solution of calcium bicarbonate is heated, a precipitate of calcium carbonate (CaCO3) forms, carbon dioxide is evolved as a gas, and water is liberated (as a gas or a liquid, depending on the temperature). On the other hand, the reaction can go from right to left if gaseous carbon dioxide is reacted with solid calcium carbonate in the presence of water and without heat.
Vocabulary
reactant, equation, arrow, to indicate, reverse direction, condition, to heat, aqueous solution, a precipitate, to liberate.
Questions
I. Describe the structure of a chemical equation
- the reactants
- the products
- a single arrow/ a double arrow
II. How may the physical state of the substance be shown in an equation? What for is the letter delta used?
Acids, bases and salts
Exercise 1. Read the text and find the passage which contains the information about one of the most famous pH indicators.
Acids are compounds (or ions) that react with water to produce hydrogen ions (H+). Hydrogen ions account for the characteristic properties of strong acids, such as a sour taste and the ability to react with bases. Bases are compounds that yield the hydroxide ion (OH-) in water solutions. Salts are ionic compounds that are generally formed by the reaction of an acid and a base:
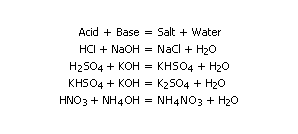
Acids are compounds that contain Hydrogen (Hydrochloric, HCl; Sulphuric, H2SO4). However, not all compounds that contain Hydrogen are acids (Water, H2O; Methane, CH4). Acids are usually compounds of non metals with Hydrogen and sometimes Oxygen. There are also organic acids. Acids are corrosive and can burn flesh and dissolve metal.
A base is a substance that gives OH- ions when dissolved in water.Bases are usually metal hydroxides (MOH). Examples include Sodium Hydroxide, NaOH, Calcium Hydroxide, Ca(OH)2. The solution of a base in water is called an alkali. Alkalis have a soapy feel and can corrode.
Acidity is measured on a scale called pH. The value of pH determines how acidic or basic a solution is. A pH of 1 is very acidic; a pH of 14 is a strong alkali. A neutral solution (or pure water) has a pH of 7. There are chemicals that change colour at different pH values. These are called indicators. One of the most famous is Litmus. This substance turns red when the pH is less than 7 (acidic) and turns blue when the pH is greater than 7 (basic).
A salt results when an acid reacts with a base. Both are neutralised. The H+ and OH- ions combine to form water. The non metalic ions of the acid and the metal ions of the base form the salt. The salt ions normally stay in solution. The salt crystalizes out when the water is removed. Some salts are insoluble. Salts can be formed in other ways. When metals dissolve in acids, a salt is formed along with Hydrogen:
Acid + Metal ----> Salt + Hydrogen
Oxides are compounds containing Oxygen combined with a single element. Oxides can be Acidic, Basic or Neutral. Acidic oxides react with water to form an acid. Basic oxides react with water to form an alkali. Most metal oxides are basic. Neutral oxides are either insoluble in water or do not form acids or alkalis when dissolved.
Exercise 2.
- give the definitions of all the inorganic compounds
- speak about their chemical properties (give your examples)
- speak about pH and its indicators
Exercise 3. Give each passage a title. Give the main idea of each passage.
Exercise 4. Render the whole text.
Unit 4 In the Laboratory
Before reading the text consult the appendix for chemistry which contains the list of the laboratory equipment.